knowledge hub
TMS for professionals
Welcome to MagVenture’s Knowledge Hub. This is where we share all the knowledge about TMS research, treatment and technology, that we’ve compiled over the years, as well as support for how to use our products. Here you’ll find links to webinars, clinical studies, training courses, “how-to” videos and more. Because we want you to access all the potential that TMS can offer.
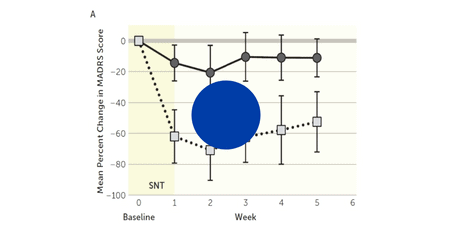
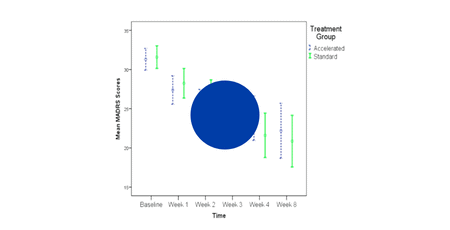
Cole et al. 2021
Stanford Neuromodulation Therapy (SNT): A double-blind randomized controlled trial
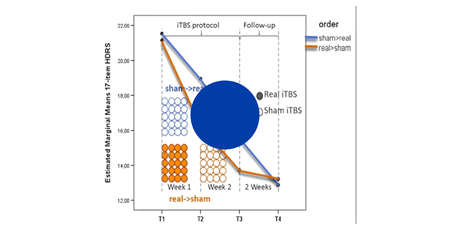
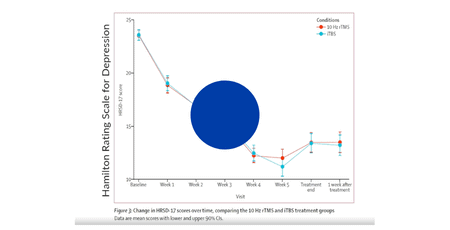
Duprat et al. 2014
Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission?

A Practical Guide to Transcranial Magnetic Stimulation Neurophysiology and Treatment Studies
by Robert Chen (Author), Paul B. Fitzgerald (Author), Daniel M. Blumberger (Author)

Transcranial Magnetic Stimulation
by Alexander Rotenberg (Editor), Jared Cooney Horvath (Editor), Alvaro Pascual-Leone (Editor)

rTMS Treatment for Depression: A Practical Guide
by Paul B. Fitzgerald (Author), Z. Jeff Daskalakis (Author)

Get in contact