product categories
Explore our products

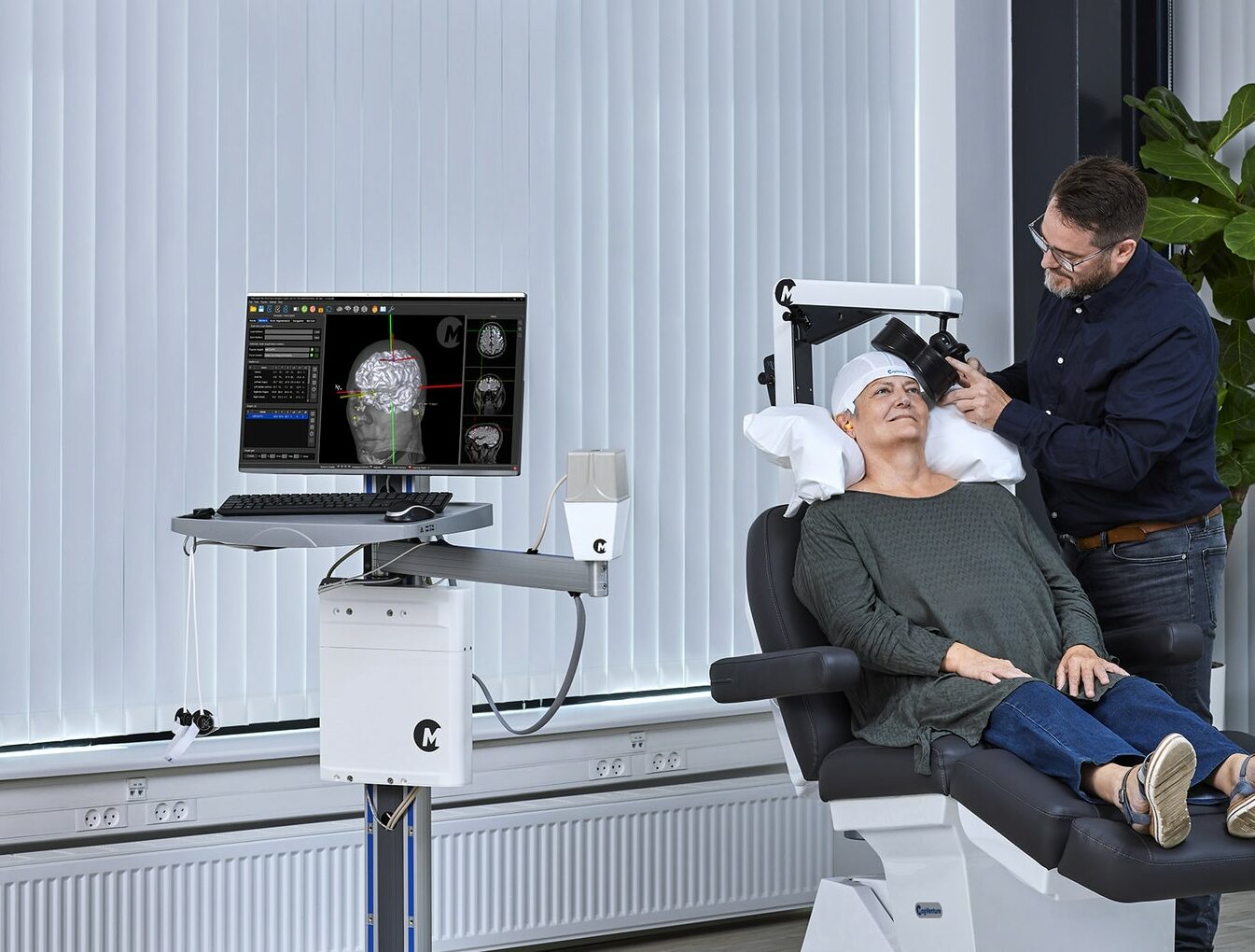
Coils
With a large selection of coils to choose from, our diverse coil selection is designed to suit your intended use in TMS research, diagnostics, and treatment. Our coils come in a range of shapes and sizes for different purposes, with features such as cooling, power control and triggering on the handle.

Stimulators
With different stimulators to choose from, our high-performing TMS solutions meet a wide range of requirements for use in research, diagnostics, and treatment. Features include defined protocols, storage and retrieval of
protocols, automatic sequence set-up, transferrable data, advanced in/out triggers for EEG, EMG, and EP equipment. Designed to ease your workflow and ensure accuracy and consistency in all TMS processes

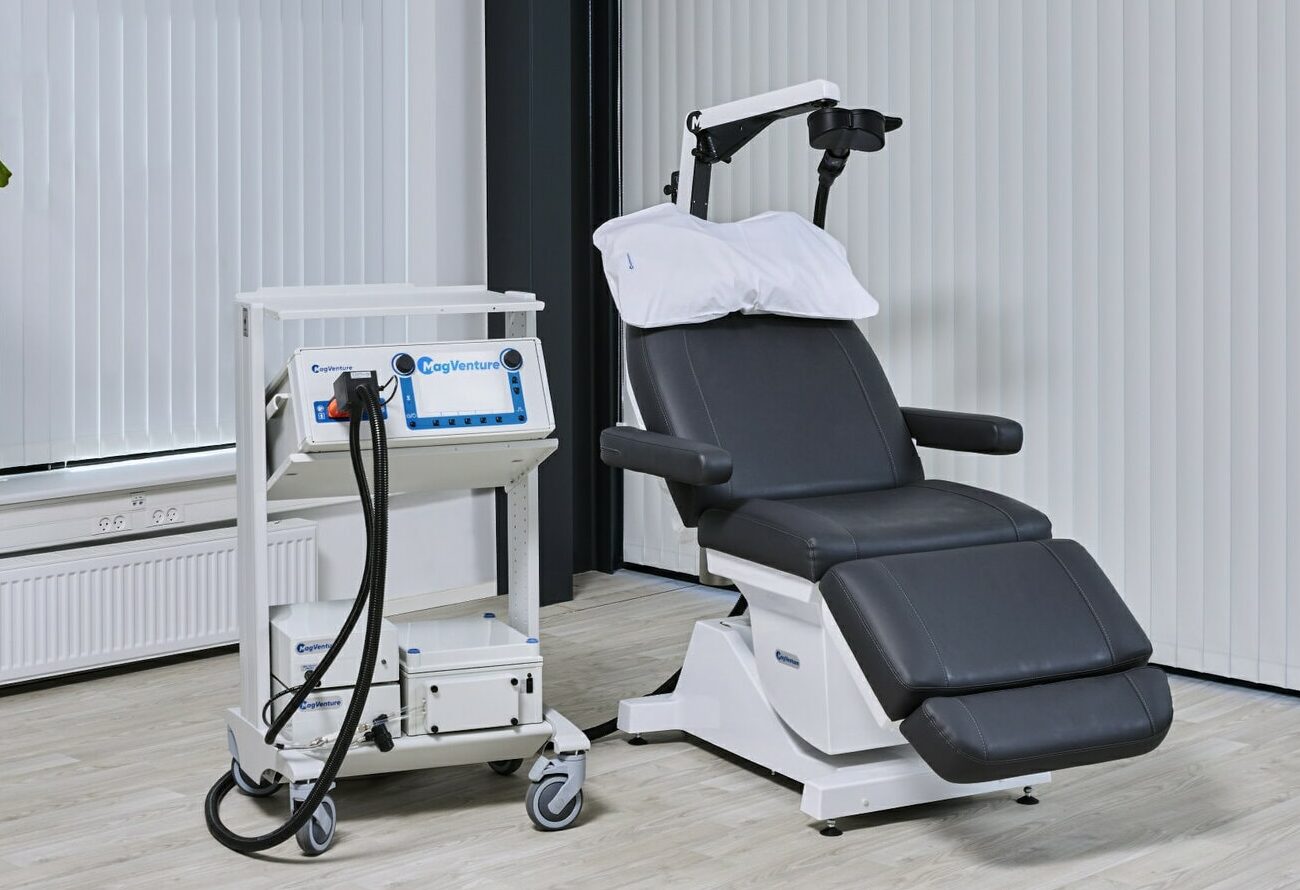
Accessories
To customize and enhance your TMS system, we have a wide range of accessories. Many of our accessories, such as our trolleys, marking plates,
mountings, and holders, have been designed to streamline your TMS process.
All our accessories are designed to suit your specific situation and requirements, and always with functionality and aesthetics in mind.
get inspired
From products to packages
Multiple combinations
It can be a bit difficult to see through all the product combinations possible. Therefore, we have gathered some of the most typical product packages within different TMS areas that can function as inspiration to you.
Professional advice
Our packages should be used as inspiration for further dialogue with one of our experts. Together, we will find the optimal combination of products for your purpose.
Support and training
As always, when you purchase a MagVenture package, you will gain access to a repository of comprehensive training videos, resources, and support information.

Get in contact