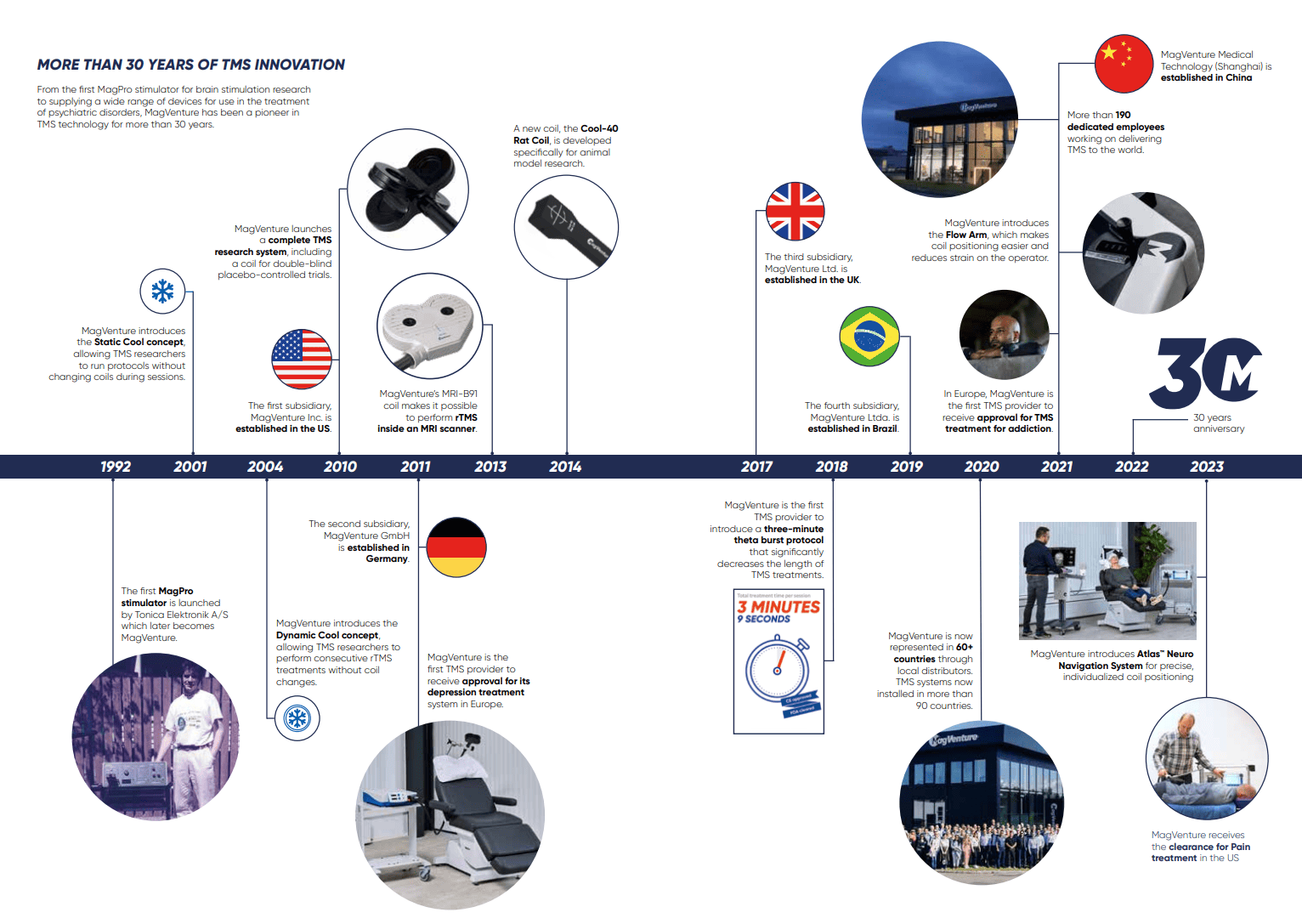
MagVenture is a market-leading manufacturer of non-invasive Transcranial Magnetic Stimulation (TMS) systems. Privately-owned and headquartered in Denmark, MagVenture has been pioneering cutting edge TMS solutions for more than 30 years. MagVenture’s TMS systems are used for a wide range of applications in both research and treatment in the fields of psychiatry, neurophysiology, neurology, cognitive neuroscience, and rehabilitation.
MagVenture’s vision is to offer new hope to patients by transforming how mental health and physical conditions are treated.
Our mission is to pioneer safe, effective, and reliable magnetic stimulation therapies, offer innovative and versatile research solutions and work to expand access to magnetic stimulation worldwide.
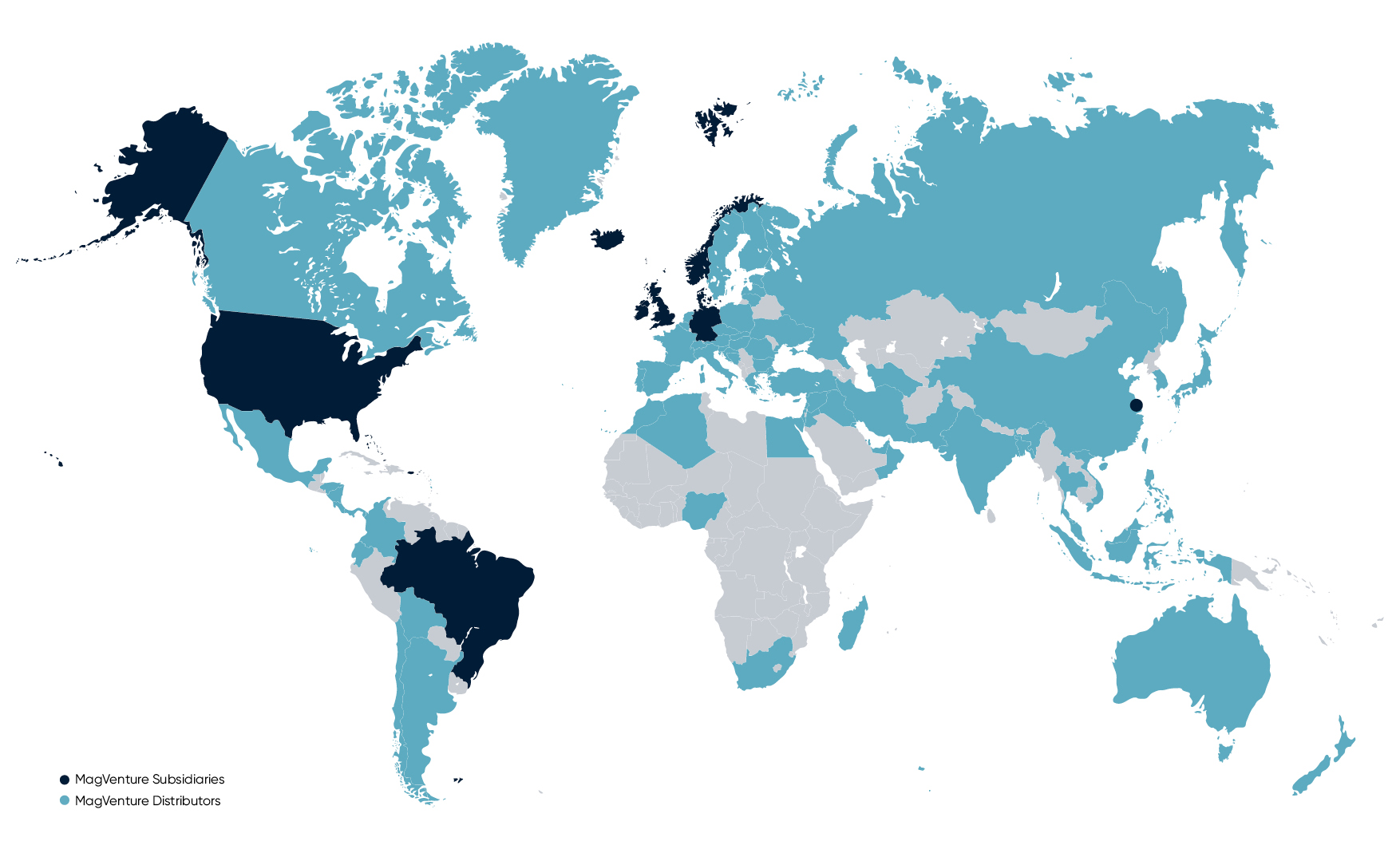
MagVenture’s products are available on the global market through direct sales subsidiaries in the USA, Germany, the UK, China, and Brazil, and through a global network of distributors.