transforming mental health
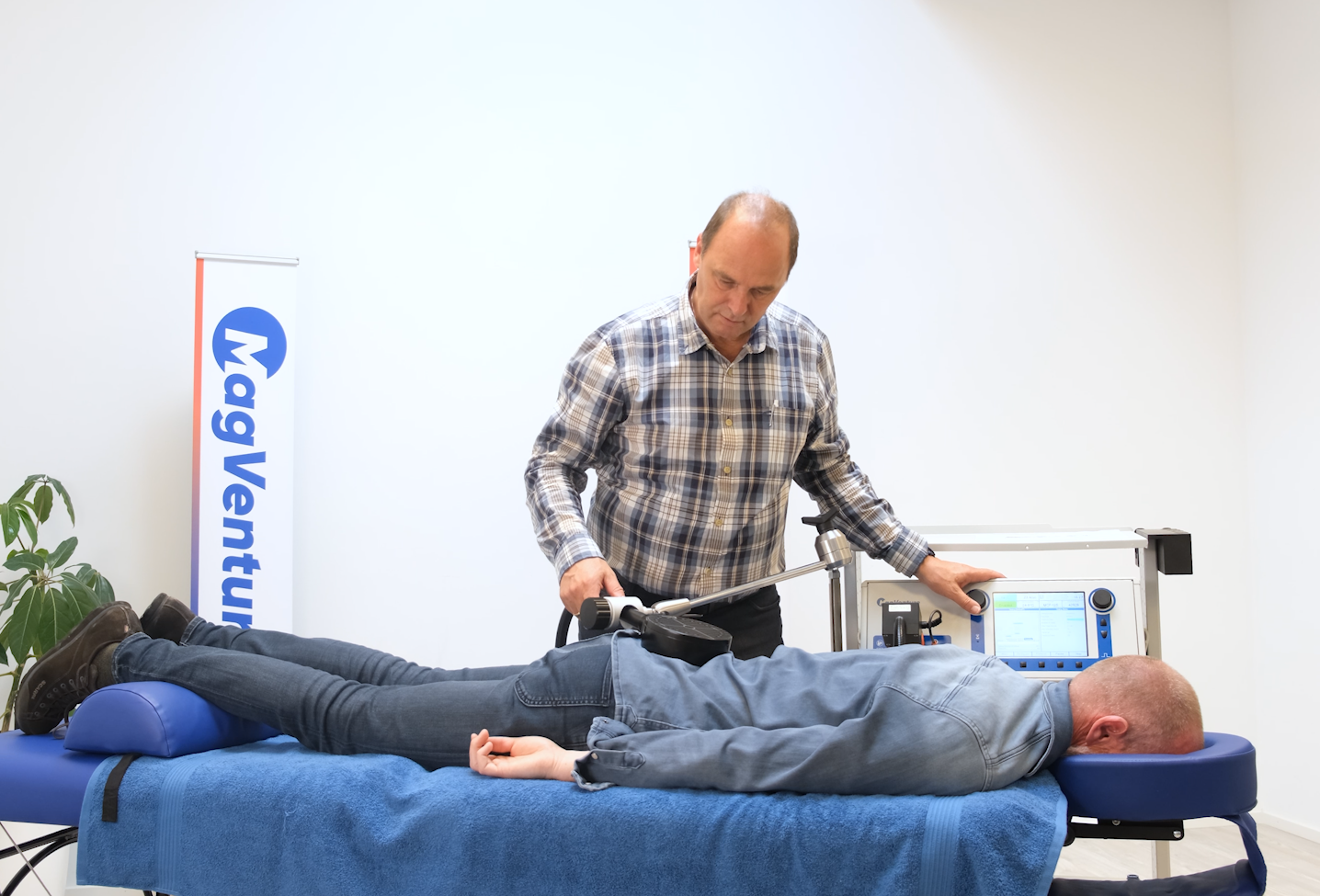
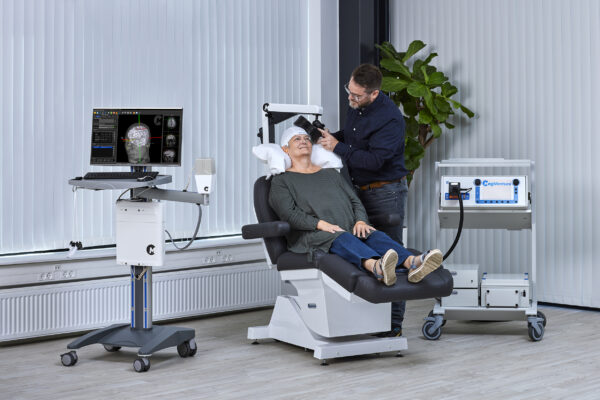
MagVenture TMS machines give new hope to patients
GROUNDED IN RESEARCH, COMMITTED TO TREATMENT, FOCUSED ON RESULTS
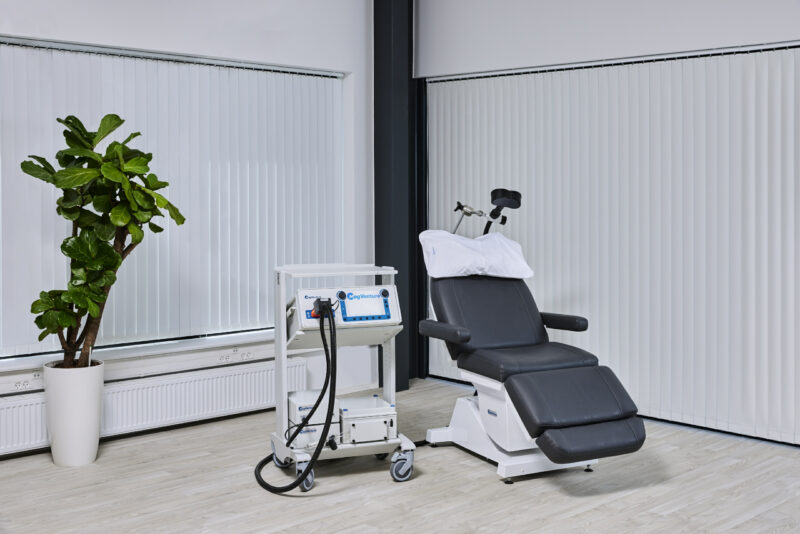
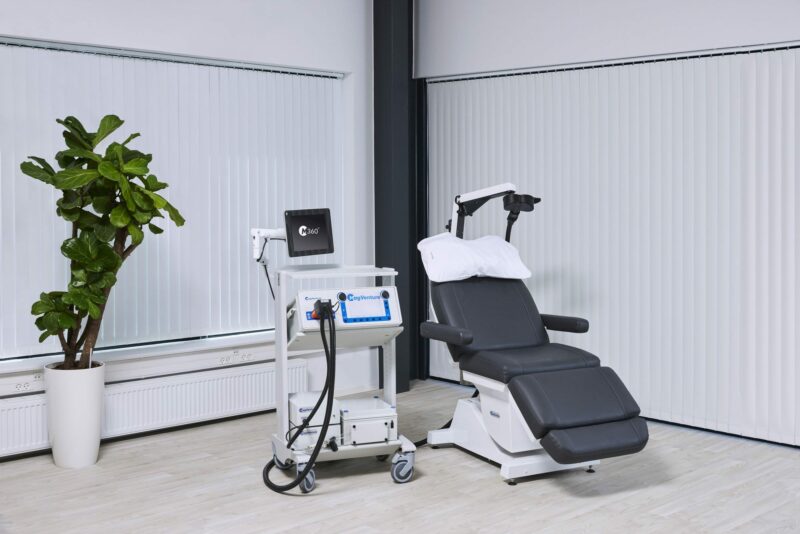
MagVenture is a TMS machine manufacturer.
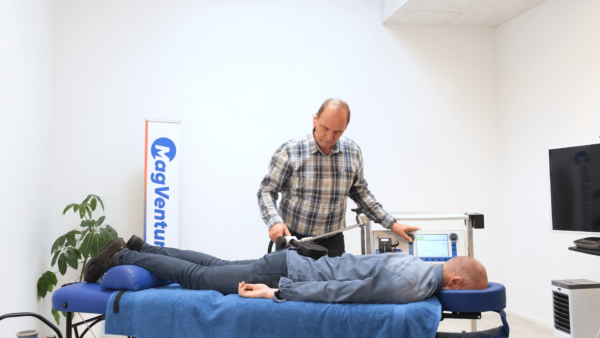
At MagVenture we are passionate about groundbreaking technology that helps improve people’s lives. That’s why we’ve been pioneering non-invasive Transcranial Magnetic Stimulation (TMS) technology for more than 30 years.
Since the earliest days of TMS, we’ve worked with thousands of researchers to expand their knowledge of the human brain. We have collaborated with leading neuroscientists to advance the fields of psychiatry, neurophysiology, neurology, neuroplasticity, cognitive neuroscience, and rehabilitation.
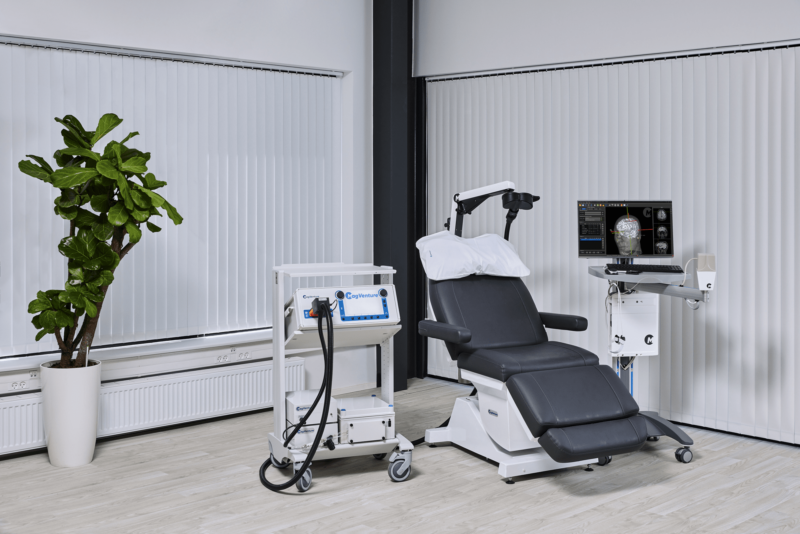
After many years grounded in the TMS field, we applied our expertise from research and diagnostics to develop clinical TMS systems. More recently we have added Magnetic Peripheral Nerve Stimulation (mPNS) solutions as well.
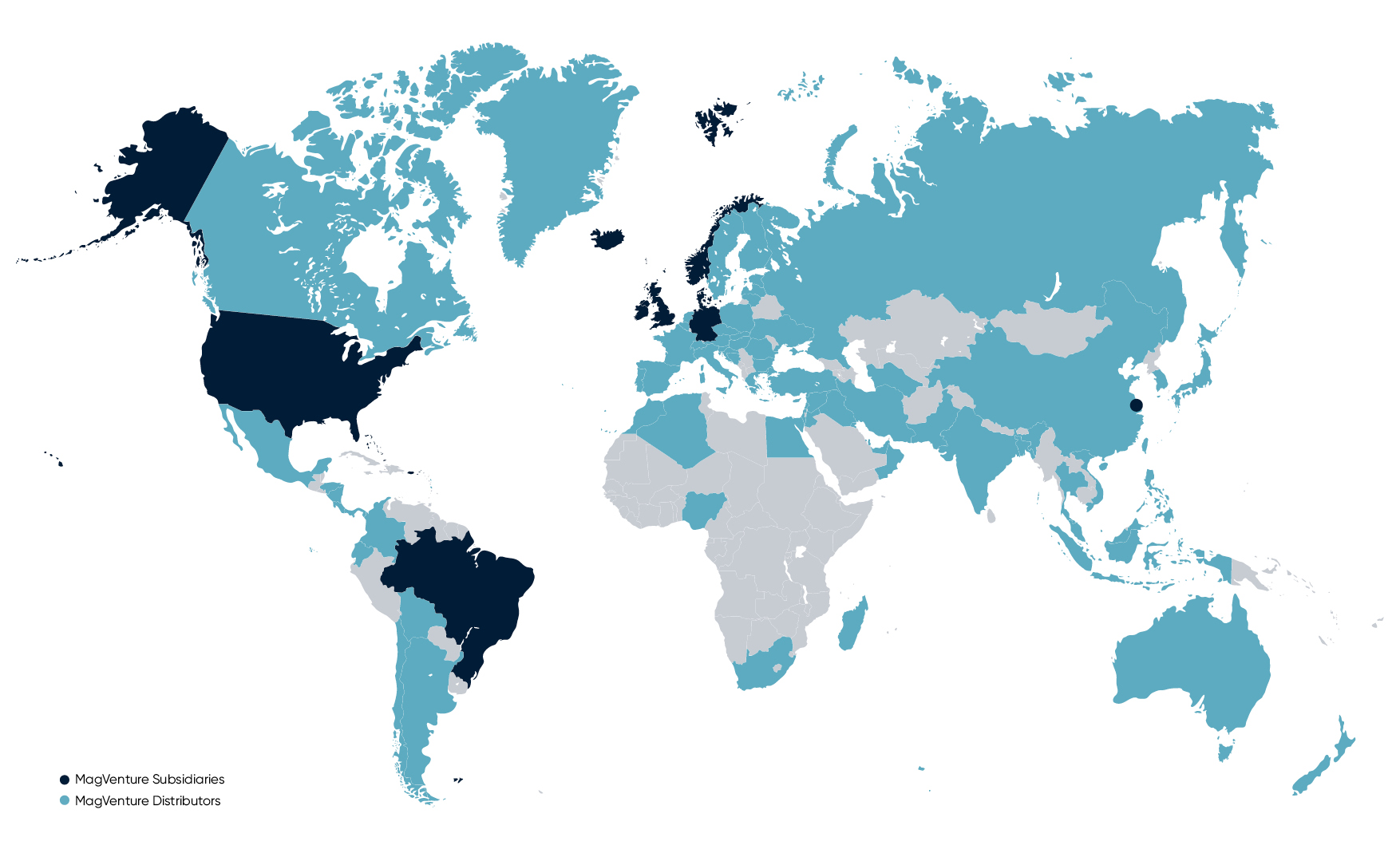
MagVenture is proud to be a privately-owned Danish company. Our products are designed, developed, produced, and quality tested in Denmark to the highest standards. And with subsidiaries in the USA, Brazil, the UK, China, and Germany, and a distribution network in more than 70 countries – our reach is global.
Find the right information
Who are you?

Psychiatrist
Are you experiencing 30% of your patients with Major Depressive Disorders not responding to traditional medication treatment?

Clinic administrator
Do you want to increase your revenue streams and enhance the clinic’s reputation as a provider of cutting-edge, evidence-based treatments?
MagVenture machines applications

Talk to a TMS expert